If the instant of an article, you get under the skin of Heizenberg ?
Come dive into the world of Walter White, the hero of the series Breaking Bad to better understand the chemistry of the 3 most known precious metals: Gold, Silver and Platinum !
In this article, we discuss simple and synthetically the following aspects :
- Chemical Symbol
- Atomic number
- Density
- Fusion point.
- Special Features
Chemical Symbol

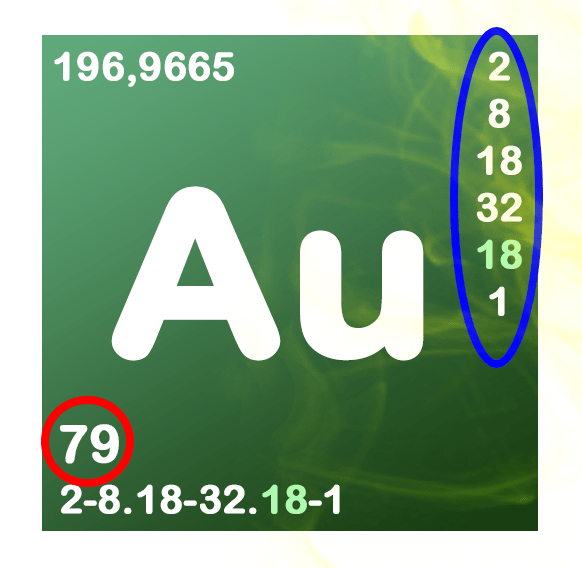
Gold, silver and platinum are presented as above in the periodic table of elements. However, their chemical symbols hadn’t been agiven by chance. They come from their etymology !
- Au the name comes from Latin Aurum, pointing gold.
- Ag as it comes from the Latin Argentum name designating money.
- Pt comes from the Spanish Platina, meaning « little silver », the name given to this metal by the Spaniards when they arrived in Colombia.
Atomic Number

It corresponds to the number of protons making up the nucleus of the atom (red). The number of protons also corresponds to the number of electrons orbiting around the nucleus of the atom.
We can therefore deduce that the atom is made up of :
- 79 protons for gold
- 47 protons for money
- 79 protons to platinum
These electrons are located in varying amounts in multiple layers (blue).
Density
The density of a metal corresponds to its weight. It’s measured by atomic mass.
To sketch the explanation, let’s take a simple example. Imagine three containers of 1 liter capacity (a bottle for example), each filled differently with our 3 precious metals in the liquid state.
Given the density differences, and for the same volume (1 liter), we get the following observation on the scale.
- Gold : we would have a weight of 19.3 kg (For the record, a cube with a ton of gold would measure only 37cm of side)
- Silver : we would have a weight of 10.50 kg
- Platinum : we would have a weight of 21.45 kg
Melting Points
The melting point corresponds to a temperature at which an item passes from the solid state to the liquid state. This temperature differs depending to metals.
- Gold : 1064 ° C.
- Silver : 962 ° C.
- Platinum : 1772 ° C.
The materials whose melting points are very high like platinum require specific material to melt, such as welding torch for example.
Special Features
Gold
- Easily malleable. If we flattened one ounce of gold could cover a surface of 7.6 square meters !
- Very good conductor of heat and electricity. Many electronic components are plated with a thin layer of gold.
- Almost insensitive to oxidation because of inertness. Hence the saying gold is eternal.
- Non-magnetic (not loving).
Silver

- Better conductivity than copper. Used in electronics.
- Can be attacked by sulphites (silver sulphide). Hence the noircessement cutlery and jewelry.
- Silver salts are photosensitive. The metal used in photography.
Platinum
- Very malleable and ductile as gold.

- Interacts with numerous molecules, making a powerfull catalyst.
- Highly resistant to corrosion at room temperature.
More news about gold on
[button type= »info » target= »_self » link= »http://orobel.biz/en/info/news.html » icon= »info-sign »]Orobel.biz[/button]